Welcome to the Jungle: Living Materials for Construction
Concrete is a huge source of emissions, but biotech could make it carbon negative by harnessing Microbially-Induced Carbonate Precipitation.
Cause life
Must be somewhere to be found
Instead of concrete jungle
-Bob Marley and the Wailers
Whether you consider it an architectural triumph or a drab eyesore, concrete is everywhere. The world produces about 30 Gt of it every year, making concrete the most-used building material by far. [1] To put that in context, global crop production [2] and oil consumption [3] are 9 Gt/yr and 5 Gt/yr, respectively.
Concrete is also a major contributor to global warming. Producing cement, one of the key ingredients in concrete, is responsible for approximately 8% of annual greenhouse gas emissions. These emissions can't be mitigated by switching to renewable energy, making them tough to abate. However, the massive scale of concrete production also creates intriguing possibilities for carbon sequestration. We need to remove gigatons of CO2 and concrete is one of the few materials that we manipulate on a gigaton scale, so can we use it to make construction net negative?
But wait, isn’t this a biotech newsletter? What does concrete have to do with biology? To find out, let’s look at how concrete is made.
Concrete consists of aggregates (i.e. rocks) mixed with cement, which binds them together. Cement is produced by mixing limestone (a.k.a calcium carbonate, or CaCO3) with clay silicates and heating them to 2,700 ℉, which yields CO2 and a solid intermediate called clinker in the following reactions [4]:
Limestone decomposes into belite and carbon dioxide.
Calcium carbonate decomposes into lime and carbon dioxide.
\(\mathrm{CaCO}_3 \rightarrow \mathrm{CaO} + \mathrm{CO}_2\)Belite and lime form alite.
\(\mathrm{Ca}_2\mathrm{SiO}_4 + \mathrm{CaO} \rightarrow \mathrm{Ca}_3\mathrm{SiO}_5\)
Then the clinker (alite and belite) is mixed with crushed gypsum, fly ash, and other “Supplementary Cementitious Materials” (SCMs) to produce cement. Cement is mixed with sand and gravel to produce concrete.
The concrete is either cast into pre-made bricks at the factory or poured on-site. Finally, freshly-poured concrete hardens through a process called “curing,” where calcium silicates react with water to form calcium hydroxide crystals [5]. This process, which requires maintaining the concrete at an optimal level of moisture for multiple weeks, is essential for concrete to reach its full strength.
Thus there are two types of emissions from cement production: those from the energy to produce heat (~40% of emissions), and those released when limestone becomes lime (>=50%) [6]. High-heat processes are notoriously hard to electrify, and the chemical emissions aren’t energy-related at all, so this is a situation where solar panels can’t save us. The graphic below illustrates the entire cement production process, from sourcing raw materials to transporting the finished product. As you can see, the heat and chemistry during clinker production account for the vast majority of CO2 emitted.
The cement production process. Most emissions come from clinker production, where fossil fuels are burned to generate heat and limestone releases CO2. Source: McKinsey and Co., “Laying the foundation for zero-carbon cement.” 2020.
https://www.mckinsey.com/industries/chemicals/our-insights/laying-the-foundation-for-zero-carbon-cement#
How can biology make this process more sustainable? There are two main approaches:
Use photosynthetic microbes to make limestone with CO2 from the atmosphere. Then burning it is net-zero, and building with it directly is carbon negative!
Use biologically-fixed carbon (wood, polymers, ash) as SCMs, sequestering carbon.
Biological SCMs could be very diverse, and they’re part of a larger conversation about carbon-negative SCMs. Research is underway to mix clinker with everything from biochar [7] to the lignin-rich byproducts of biofuel production [8]. On the other hand, recent research on abiotic CO2 utilization in concrete casts doubt on the true carbon impact after emissions for the extra processes are accounted for [9]. This will be a recurring theme in our newsletter: you can’t truly know the impact of some shiny new technology without careful Life Cycle Analysis (LCA), which quantifies emissions for the whole process from “cradle to grave.”
For the rest of this post, we’re going to focus on approach #1 because it’s driven by one powerful biological process: microbially-induced carbonate precipitation (MICP). Below, we’ll describe the science of MICP and the techno-economic challenges of competing with traditional concrete.
The science
Carbonate precipitation is a chemical process where water-dissolved CO2 binds to a cation (a positively charged atom like Ca+ or Mg+) to form solid carbonate. Carbonate precipitation seems like a promising form of carbon dioxide removal (CDR): the CO2 gets stably sequestered into a solid, and the process is one of the few CO2 transformations that’s thermodynamically favorable; i.e. CO2 “wants” to form a carbonate, whereas making most other molecules takes energy. If I added calcium ions to a glass of water and walked away, some atmospheric CO2 would form carbonate without my having to lift a finger.
The two problems are capacity and speed. You might remember from your high school chemistry class that a chemical reaction A ↔ B stops when the system reaches a balance of A to B, which depends on their amounts and intrinsic energies. This is called “equilibrium.” For carbonate precipitation in a small body of water, the reaction quickly reaches equilibrium and stops, so no more carbon dioxide is pulled out of the air. In the ocean, we have a different problem. Typically we keep reactions going by removing products, so the system creates more carbonates to restore equilibrium. But in the ocean, the volumes are so huge that it’s difficult to drive down the level of dissolved CO2 enough to favor capturing more atmospheric CO2 (although decreasing ocean acidification is certainly a good thing on its own!).
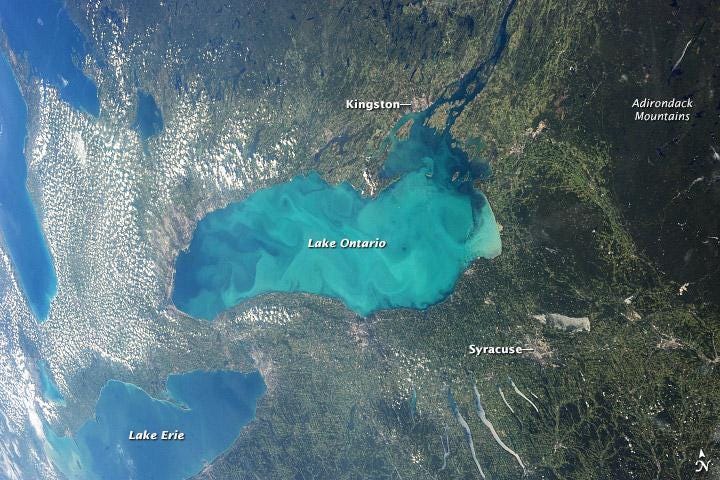
That’s where microbes come in: diverse organisms drive the system out of equilibrium and induce carbonate precipitation as a byproduct of their metabolism. This can be seen most strikingly in “whiting events,” where algal blooms are believed to induce so much precipitation that it’s visible from space:
A 2013 whiting event in Lake Ontario was visible from the space. Source: International Space Station Expedition 36 Crew https://earthobservatory.nasa.gov/images/81952/whiting-event-lake-ontario
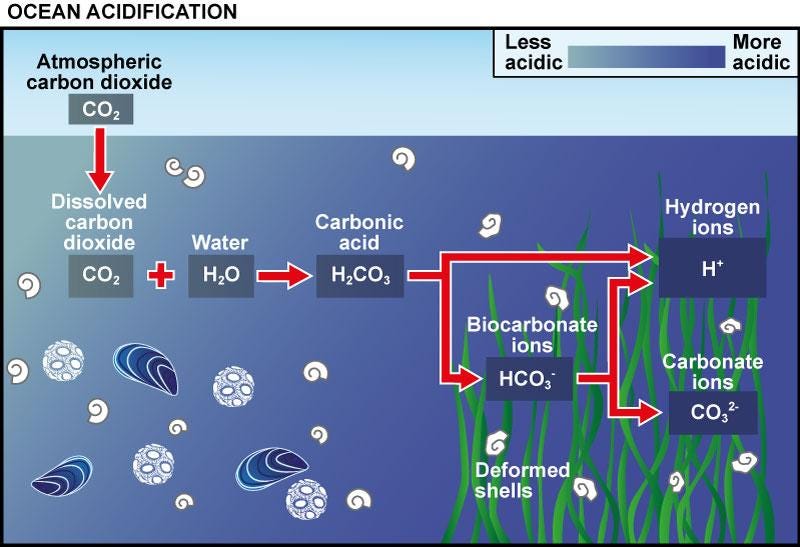
What are the microbes doing? To answer this question, we need to understand the fate of carbon dioxide in water, illustrated in the cartoon below. When CO2 dissolves in water, it can form carbonic acid, H2CO3, which dissociates to form bicarbonate, HCO3-. Then bicarbonate can dissociate again to form carbonate, CO32-, which binds to Ca+ or Mg+ and precipitates out of solution. (To see how Ca+ and extra bicarbonate reach the ocean, look into enhanced rock weathering, another important area of CDR [10].)
Dissolved CO2 takes on many forms, depending on the concentration of carbon and protons (H+). In photosynthetic MICP, microbes raise the pH to make bicarbonate dissociation more favorable. Source: UK Ocean Acidification Research Programme https://www.oceanacidification.org.uk/
The microbes influence this process in two ways:
They shift the balance of H+ (i.e. the pH) and dissolved carbon to make precipitation more favorable.
They provide physical nucleation sites to boost precipitation.
Microbial metabolism is wildly diverse, with different microbes specializing in using different molecules for energy. It turns out that carbonate precipitation is favored by many of these metabolisms – including photosynthesis, ureolysis, denitrification, ammonification, sulfate reduction, and methane oxidation – making MICP a phenomenon with potentially wide applicability.
For the purposes of climate biotech, we’re going to focus on photosynthetic organisms, in particular cyanobacteria and microalgae (metabolism nerds can check out [11] for a more general review). These phytoplankton are appealing for biotech because they grow on light and CO2, lowering the feedstock costs and increasing the carbon removal potential, and because they include model organisms that biologists have experience working with. For example, the cyanobacteria Synechocystis is a well-studied model organism with a fully sequenced genome, and the interest in algal biofuels has led to serious analysis and technological learning to scale [12]. As a result, cyanos and algae are more ready to scale than some other organisms.
The first way phytoplankton contribute to precipitation is by taking up bicarbonate (HCO3-) and releasing OH-. This raises the pH, making carbonate precipitation more favorable. Why? Remember, the equation for carbonate precipitation is
So, it requires freeing an H+. Microbially-produced OH- binds to H+ to produce water, reducing the concentration of protons and driving the reaction in the forward direction.
A photosynthetic cell takes up CO2 and bicarbonate, and releases OH-, driving carbonate precipitation. Adapted from [11].
The second way phytoplankton promote precipitation is by catalyzing it at the cell surface [13]. The mechanisms here are poorly understood, but it’s believed that some combination of surface proteins and saccharide polymers (exopolymeric substances, or EPS) can trap cations and form microenvironments where carbonates form. However, the physical mechanisms and genetic control for this are still pretty mysterious.
Biotech challenges
If we want to scale MICP to remove gigatons of CO2, first we need to engineer cyanobacteria to precipitate construction-scale amounts of CaCO3. There are some processes that have been hyperoptimized by evolution and industrial biotech to the extent that future gains are likely to be hard-won. This is not one of them. Cyanos didn’t evolve to precipitate, so there’s no reason to believe that we can’t engineer them to be better. Moreover, very little effort has been devoted to engineering MICP relative to applications like industrial fermentation. The downside is that we’re starting almost from scratch. We found three published precipitation rates, in mg of CaCO3/L/hour: 37.5 [14], 2 [15], and 30 [16].
As you can see, not much has been done to quantify the status quo, much less optimize it. Here are the areas synthetic biologists and biotech could explore to get that rate up:
Engineer photosynthesis and transporters to optimize cation/OH- balance. Encouragingly, Jiang et al. 2013 [16] were able to boost precipitation rates by 50-100% by knocking out a single Ca2+/H+ exchanger.
Engineer surface proteins and EPS to optimize carbonate microenvironments.
Optimize culture conditions and reactor design for Synectostysis or microalgae to collect the most CaCO3 at the lowest cost, enabling production at the million-liter scale.
As a first step, biotechnologists should prioritize developing scalable, high-throughput platforms to measure carbonate precipitation. This would allow scientists to screen a library of mutants and identify promising genes to target, unlocking the efficiencies necessary to scale up.
Market analysis
At 30 Gt every year, the scale of concrete production is staggering. We’ve all seen concrete mixers around construction sites because it is the most widely used construction material [17]. Interestingly, despite its scale, concrete production is often a local business. Concrete is expensive to transport and has to be set on site, favoring local distributors and manufacturers. The exact make-up of concrete can also vary by available resources and desired properties. Unfortunately, this fragmentation across producer and use cases makes it hard to find aggregate numbers on revenue, market size, and utilization.
Statistics on cement are more readily available. Annual production of Ordinary Portland Cement is 3.5 billion tons, leading to around 2.1 Gt of emissions each year. Total cement production is about 4 billion tons, contributing an estimated 2.8 Gt of emissions [18]. China is the biggest producer of cement in response to a growing economy and appetite for construction, accounting for almost 60% of total production in 2015 [19]. The same year McKinsey estimated the global market valuation as $450 billion [20].
Cement production increased globally about 25% between 2010 and 2015. Production in China accounted for about two-thirds of that increase, highlighting the global challenge of reducing cement emissions. Production in the US has also increased in relative terms, though the absolute tonnage is a small share of global production.
Source: Trends in global CO2 emissions: 2016 Report, The Hague: PBL Netherlands Environmental Assessment Agency, https://www.pbl.nl/sites/default/files/downloads/pbl-2016-trends-in-global-co2-emisions-2016-report-2315_4.pdf
Cement is predominantly used for construction with concrete. The public sector is a large consumer. Roads and bridges are a little under 20% of American cement consumption (see the sector-by-sector breakdown below). In North America, public works projects are about a third of consumption [21]. In addition to being durable, cement has achieved its ubiquity by being cheap. The National Ready Mixed Concrete average surveyed price of a cubic yard of concrete was $125 [22]. Various types of steel trade above $1,000 per ton. (steel is a traded commodity, making price estimates easier). At a density around 0.3 lb/in3, steel is about $7000 per cubic yard, an order of magnitude more expensive.
Cement is consumed by key infrastructure sectors for construction, such as roads & bridges, airports, and electrical grids. These public-serving sectors mean concrete is important to maintaining the way we live, but also mean we as consumers can have a say in what types of materials are used in construction.
Source: PCA Cement Outlook, Spring 2022 https://www.cement.org/docs/default-source/market-economics-pdfs/ed-sullivan's-spring-forecast-2022.pdf?sfvrsn=506cffbf_2
The scale of concrete makes it an exciting area for disruption. On the flip side, its low price makes it difficult to compete with, especially for less mature technologies. Nevertheless, some start-ups have already taken on the challenge of introducing low-carbon concrete using biology.
Two companies, Minus Materials and Prometheus Materials, have been spun out of technology from Will Srubar’s group at CU Boulder. Minus Materials seems to be focused on speeding up microalgae-MICP to produce limestone, which can be dropped into the standard cement production process. They claim they can “shorten the formation of limestone quarries from millennia to minutes while sequestering and storing carbon dioxide in the process.” If successful, this would make the chemical emissions from burning limestone (remember, around 50% of emissions) net-zero.
Prometheus is focusing on “engineering living biomaterials.” While the technical details on the website are vague, academic papers indicate that this entails mixing cyanobacteria with sedimentary material. As the cyanobacteria precipitate carbonates, the material hardens. However, since the cyanobacteria remain alive, the material can repair itself or be used to seed future materials. Srubar’s first living building materials papers demonstrated a living sand-hydrogel scaffold with Synechococcus sp. PCC 7002 [23]. This cyanobacterial strain performs photosynthesis, creating a carbon-capturing material. A second paper [24] improved on the bacterial viability of the first by adding trehalose to the mixture and also evaluated a biomaterial with E. coli using a ureolytic pathway. A patent filed in March 2020 claims the results from both papers, as well as materials with some Pseudomonas, Myxococcus, Bacillus, and Lycinbacillus strains and a variety of inorganic substrates [25]. The results in academic literature, however, did not demonstrate materials as strong as traditional concrete. The maximum compressive strength reported, 4.82 MPa, is still about three times less than the minimum concrete compressive strength 17 MPa [26].
At $50 per ton, it’s tough to compete with the price and scale of crushed limestone. However, the more specialized market for tiles and bricks offers an opportunity for bioconstruction companies to enter the market and learn to scale. This is exactly the strategy taken by Biomason, a NC-based company founded in 2012. Biomason makes tiles by mixing aggregates, microbes, and some nutrients in a cast. Then the microbes precipitate CaCO3, which binds together the aggregates as “biocement.” The tile market is about $350 billion [27], and with customers willing to spend 10-100x more per weight than for crushed limestone, one can see how the unit economics are less punishing for a new technology. Biomason is currently working to scale up production capacity from 100,000 ft²/year to 1 million ft²/year, and their recent hire of a former VP of automotive manufacturing at Tesla shows that they’re serious about scaling. Apart from their tiles, they also have pilot projects with the US military and the state of North Carolina on soil stabilization and self-healing marine infrastructure, respectively.
Technical details about private ventures like Biomason are scarce. But governments, as large consumers of concrete, are also naturally invested in optimizing production and tend to divulge more details on their funding. There is a wealth of information available about the nonprofit BioZement project, funded by the Research Council of Norway [28-30]. Like Biomason, BioZement aims to use MICP to make CaCO3 bricks. They have a two-step process. First, they use a bacterial strain that consumes glucose and secretes acid to dissolve crushed limestone into Ca+ and HCO3- ions. Then they feed the slurry into a mold with aggregates, urea, and a second strain that induces precipitation by breaking down urea. The result is a biocement brick with a compressive strength comparable to C8/10 concrete, suitable for paving and other low-strength applications.
Most importantly for us, they perform a detailed TEA (technoeconomic analysis) and LCA to understand the economic viability and environmental impact of their technology [30]. This gives insights into cost drivers, like the glucose and urea to feed the bacteria. Urea hydrolysis also produces GHGs, which must be captured or factored into the overall environmental impact. They find that BioZEment bricks would be more expensive than conventional bricks, but that their technology has a greater opportunity to compete in the markets for precast and onsite production, showing the importance of nuanced market segmentation in determining the commercial viability of a new technology.
The United States also actively funds research into carbon-negative concrete. Biomason and the CU Boulder spinouts are both supported by the Department of Energy’s tech commercialization arm, ARPA-E. In addition to the above, ARPA-E’s HESTIA program is also funding projects out of UPenn, the National Renewable Energy Laboratory, Wisconsin Madison, and Texas A&M [31]. Many of these are taking approach #2, integrating organic matter into the concrete production. This process locks away carbon for a long time in the building material. Texas A&M, for example, is working on 3D printing with hempcrete, another promising low carbon biomaterial.
Takeaways:
Like it or not, the concrete jungle is here to stay. Concrete’s high strength and low cost made the past century of urbanization possible, and it continues to spread across the planet. That scale has made it a huge source of GHG emissions but also creates massive opportunities to sequester carbon dioxide. Phytoplankton like cyanobacteria and microalgae can convert atmospheric CO2 into calcium carbonate solids for low- or negative-carbon materials. Engineering those microbes with the modern tools of synthetic biology and integrating them into construction could help make buildings carbon negative, so that one day the concrete jungle swallows up CO2 just like the real one.
Next time: Tune in to find out how biology can help us achieve grid-scale electricity storage!
References
[1] Paulo JM Monteiro, Sabbie A Miller, and Arpad Horvath. “Towards sustainable concrete”. In: Nature materials 16.7 (2017), pp. 698–699. url: https://www.nature.com/articles/nmat4930.
[2] FAO. “World food and agriculture—statistical yearbook 2020”. In: World Food and Agriculture-Statistical Yearbook (2020). url: https://doi. org/10.4060/cb1329en.
[3] IEA. “Oil 2021”. In: IEA Reports (2021). url: https://www.iea.org/ reports/oil-2021.
[4] Dylan Moore. Clinker Thermochemistry. 2019. url: https://www.cementkilns.co.uk/ckr_therm.html.
[5] UIUC Department of Materials Science and Engineering. Concrete: Scientific Principles. 2019. url: http://matse1.matse.illinois.edu/ concrete/prin.html.
[6] Johanna Lehne and Felix Preston. “Making Concrete Change: Innovation in Low-Carbon Cement and Concrete”. In: Chatham House: Cambridge (2018), p. 138. url: https://www.chathamhouse.org/2018/06/makingconcrete-change-innovation-low-carbon-cement-and-concrete.
[7] Liang Chen et al. “Biochar-augmented carbon-negative concrete”. In: Chemical Engineering Journal 431 (2022), p. 133946. url: https://doi.org/10.1016/j.cej.2021.133946.
[8] Wale Odukomaiya. High-Performing Carbon-Negative Concrete Using LowValue Byproducts From Biofuels Production. 2022. url: https://arpae.energy.gov/sites/default/files/2022-10/D3%29%20NREL.pdf.
[9] Dwarakanath Ravikumar et al. “Carbon dioxide utilization in concrete curing or mixing might not produce a net climate benefit”. In: Nature communications 12.1 (2021), p. 855. url: https://doi.org/10.1038/ s41467-021-21148-w.
[10] Johannes Lehmann and Angela Possinger. Removal of atmospheric CO2 by rock weathering holds promise for mitigating climate change. 2020. url: https://www.nature.com/articles/d41586-020-01965-7.
[11] Tingting Zhu and Maria Dittrich. “Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review”. In: Frontiers in bioengineering and biotechnology 4 (2016), p. 4. url: https://doi.org/10.3389/fbioe.2016.00004.
[12] Ryan Davis et al. Process design and economics for the production of algal biomass: algal biomass production in open pond systems and processing through dewatering for downstream conversion. Tech. rep. National Renewable Energy Lab.(NREL), Golden, CO (United States), 2016. url: https://www.nrel.gov/docs/fy16osti/64772.pdf.
[13] Nina A Kamennaya et al. “Cyanobacteria as biocatalysts for carbonate mineralization”. In: Minerals 2.4 (2012), pp. 338–364. url: https://doi. org/10.3390/min2040338.
[14] Kimberly K Yates and Lisa L Robbins. “Production of carbonate sediments by a unicellular green alga”. In: American Mineralogist 83.11 (1998), pp. 1503–1509. url: https://doi.org/10.2138/am-1998-1111.
[15] Brady D Lee, William A Apel, and Michelle R Walton. Whitings as a Potential Mechanism for Controlling Atmospheric Carbon Dioxide Concentrations– Final Project Report. Tech. rep. Idaho National Lab.(INL), Idaho Falls, ID (United States), 2006. url: https://doi.org/10.2172/911640.
[16] Hai-Bo Jiang et al. “Inactivation of Ca2+/H+ exchanger in Synechocystis sp. strain PCC 6803 promotes cyanobacterial calcification by upregulating CO2-concentrating mechanisms”. In: Applied and Environmental Microbiology 79.13 (2013), pp. 4048–4055. url: https://doi.org/10.1128/ AEM.00681-13.
[17] James Mitchell Crow. “The concrete conundrum”. In: Chemistry World (2008). url: https://www.rsc.org/images/Construction_tcm18114530.pdf.
[18] Jonathan Watts. “Concrete: the most destructive material on Earth”. In: The Guardian (2019). url: https://www.theguardian.com/cities/ 2019/feb/25/concrete-the-most-destructive-material-on-earth.
[19] Jos G.J. Olivier et al. “Trends in global CO2 emissions: 2016 Report”. In: (2016). url: https://www.pbl.nl/sites/default/files/downloads/ pbl-2016-trends-in-global-co2-emisions-2016-report-2315_4. pdf.
[20] Michael Birshan et al. “The cement industry at a turning point: A path toward value creation”. In: McKinsey and Company: Chemicals (2015). url: https://www.mckinsey.com/industries/chemicals/our-insights/ the-cement-industry-at-a-turning-point-a-path-toward-valuecreation#/.
[21] IN York and I Europe. “Concrete needs to lose its colossal carbon footprint”. In: Nature 597.7878 (2021), pp. 593–594. url: https://www.nature.com/articles/d41586-021-02612-5
[22] Concrete Price Considerations - Cost of Concrete. 2023. url: https: //www.concretenetwork.com/concrete-prices.html.
[23] Chelsea M Heveran et al. “Biomineralization and successive regeneration of engineered living building materials”. In: Matter 2.2 (2020), pp. 481– 494. url: https://doi.org/10.1016/j.matt.2019.11.016.
[24] Jishen Qiu et al. “Engineering living building materials for enhanced bacterial viability and mechanical properties”. In: iScience 24.2 (2021), p. 102083. issn: 2589-0042. url: https://doi.org/10.1016/j.isci.2021.102083.
[25] International Patent WO2020/180914 A1: Living Structural Material. 2020. url: https://patentimages.storage.googleapis.com/46/f8/b6/ac69d9879b65f1/WO2020180914A1.pdf.
[26] What Is The Standard Strength Of Concrete? url: https://www.bigdreadymix.com/what-is-the-standard-strength-of-concrete/#:~:text= Usually%2C%20the%20compressive%20strength%20of,10%2C000% 20psi%20(70%20MPa).
[27] Amy Feldman. “Startup Biomason Makes Biocement Tiles, Retailer HM Group Plans To Outfit Its Stores’ Floors With Them”. In: (2021). url: https://www.forbes.com/sites/amyfeldman/2021/06/14/startupbiomason-makes-bio-cement-tiles-retailer-hm-group-plans-tooutfit-its-stores-floors-with-them/?sh=1b2304bc57c9.
[28] BioZEment 2.0. url: https://www.mn.uio.no/fysikk/english/research/projects/biozement2/index.html.
[29] Anja Røyne et al. “Towards a low CO2 emission building material employing bacterial metabolism (1/2): The bacterial system and prototype production”. In: PloS one 14.4 (2019), e0212990. url: https://doi.org/ 10.1371/journal.pone.0212990.
[30] Anders Myhr et al. “Towards a low CO2 emission building material employing bacterial metabolism (2/2): Prospects for global warming potential reduction in the concrete industry”. In: PloS one 14.4 (2019), e0208643. url: https://doi.org/10.1371/journal.pone.0208643.
[31] HESTIA—Harnessing Emissions into Structures Taking Inputs from the Atmosphere. url: https://arpa-e.energy.gov/sites/default/ files/documents/files/HESTIA%20project%20descriptions_FINAL_6.13.22.pdf.